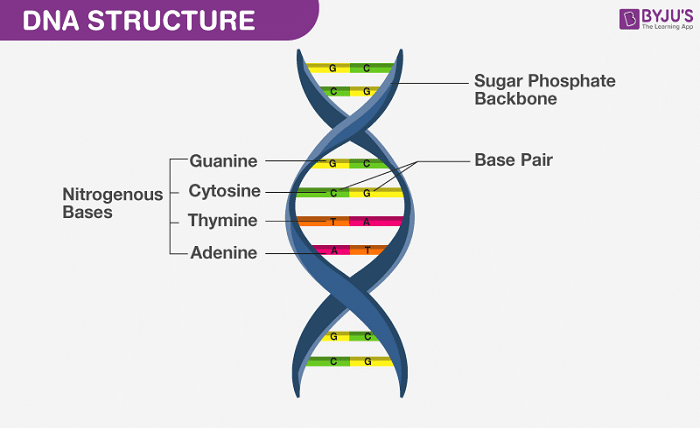
To rank the following base pairs according to their stability, we first need to understand the basics of DNA structure. DNA consists of two long strands forming a double helix, held together by base pairs. Each base pair consists of a purine (Adenine or Guanine) and a pyrimidine (Thymine or Cytosine) connected by hydrogen bonds.
The Importance of Base Pair Stability
The stability of DNA base pairs is vital for accurate replication and transcription processes. Errors in base pairing can lead to mutations, which might result in various genetic disorders. Therefore, ranking the following base pairs according to their stability is essential for understanding their role in genetic fidelity.
Hydrogen Bonding in Base Pairs
Hydrogen bonds play a crucial role in the stability of DNA base pairs. Adenine pairs with Thymine through two hydrogen bonds, while Guanine pairs with Cytosine through three hydrogen bonds. This difference in hydrogen bonding is a primary factor in ranking the following base pairs according to their stability.
Adenine-Thymine (A-T) Base Pair Stability
A-T base pairs are held together by two hydrogen bonds. This relatively lower number of hydrogen bonds makes them less stable compared to G-C pairs. When ranking the following base pairs according to their stability, A-T pairs are generally considered less stable.
Guanine-Cytosine (G-C) Base Pair Stability
G-C base pairs are connected by three hydrogen bonds, making them more stable than A-T pairs. This increased stability is crucial in regions of DNA that require high fidelity, such as coding sequences. Thus, when we rank the following base pairs according to their stability, G-C pairs top the list.
Thermodynamic Stability
Thermodynamic stability refers to the energy required to separate the base pairs. G-C pairs, with their three hydrogen bonds, require more energy to denature compared to A-T pairs. This aspect is another reason why we rank the following base pairs according to their stability with G-C pairs being more stable.
Melting Temperature (Tm)
The melting temperature (Tm) is the temperature at which half of the DNA helix denatures into single strands. DNA regions with higher G-C content have a higher Tm due to the greater stability of G-C pairs. This factor is crucial when we rank the following base pairs according to their stability.
Base Pair Stability in Different Conditions
The stability of DNA base pairs can be influenced by environmental conditions such as pH and ionic strength. G-C pairs tend to maintain their stability better under varying conditions compared to A-T pairs. Thus, when we rank the following base pairs according to their stability, G-C pairs remain more robust across different environments.
Biological Implications of Base Pair Stability
The stability of base pairs affects various biological processes. For instance, DNA regions rich in G-C pairs are often found in promoter regions of genes, where stability is crucial for proper gene regulation. Ranking the following base pairs according to their stability helps in understanding their functional roles in the genome.
Base Pair Stability in Mutations and Diseases
Mutations often occur in regions of DNA with lower stability, such as A-T rich regions. Understanding and ranking the following base pairs according to their stability can provide insights into the mechanisms behind genetic mutations and their links to diseases.
Base Pair Stability in Biotechnology Applications
In biotechnology, the stability of DNA base pairs is leveraged in various applications, such as PCR amplification and DNA sequencing. G-C rich primers are often preferred due to their higher stability. Thus, ranking the following base pairs according to their stability is critical for optimizing these techniques.
Advances in DNA Research and Base Pair Stability
Recent advancements in DNA research have provided deeper insights into the stability of base pairs. New techniques and technologies allow for more precise measurements and manipulation of DNA stability. Ranking the following base pairs according to their stability continues to be a fundamental aspect of ongoing research.
Conclusion
When we rank the following base pairs according to their stability, Guanine-Cytosine (G-C) pairs are more stable than Adenine-Thymine (A-T) pairs. This increased stability is due to the presence of three hydrogen bonds in G-C pairs compared to two in A-T pairs. Understanding the stability of DNA base pairs is essential for various biological and biotechnological applications, from genetic research to disease prevention and treatment. By ranking the following base pairs according to their stability, we gain valuable insights into the mechanisms that ensure genetic fidelity and the innovations that continue to advance the field of molecular biology.
FAQs
1.Why are G-C base pairs more stable than A-T base pairs?
G-C base pairs are more stable because they have three hydrogen bonds, compared to the two hydrogen bonds in A-T pairs.
2.How does base pair stability affect DNA replication?
Higher stability of base pairs ensures accurate DNA replication, reducing the chances of mutations and maintaining genetic integrity.
3.What is the significance of melting temperature (Tm) in DNA stability?
The melting temperature (Tm) indicates the thermal stability of DNA. Regions with higher G-C content have a higher Tm due to increased stability.
4.How does environmental pH affect base pair stability?
Environmental pH can influence hydrogen bonding and overall DNA stability, with G-C pairs generally maintaining stability better across varying pH levels.
5.Why is understanding base pair stability important in biotechnology?
Knowledge of base pair stability is crucial for optimizing biotechnological techniques such as PCR and DNA sequencing, where stability affects efficiency and accuracy.





